Activation
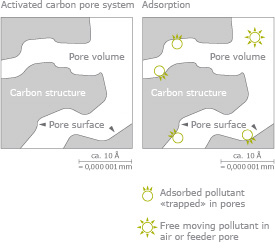
Carbon is activated by breaking down the carbon structure with steam or chemicals. This creates many fine channels, known as pores, in the carbon structure. These pores are finely grained so the polluting molecules can first penetrate them and then bond with the surface.
These purifying pores are classified as:
- Micropores up to 20 Å = 0,000’002 mm
- Mesopores 20 – 500 Å = 0,000’002 mm – 0,000’050 mm
- Macropores over 500 Å = 0,000’050 mm
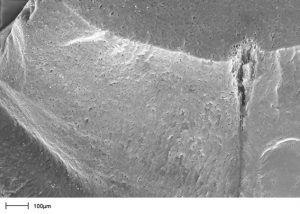

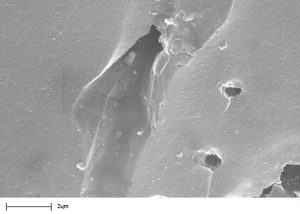
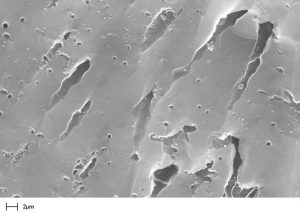

Depending on the degree of activation, activated carbons have an active internal surface area ranging from 750 m²/g to 1’600 m²/g.
Activated Carbon for Air Purification
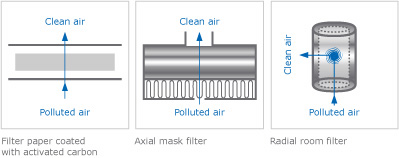
The air is forced over a carbon layer for purification. There are various types of filters; the particle sizes can range from very fine to very coarse. When describing activated carbons, the particle sizes are generally listed in US mesh.
| US mesh | 4 | 6 | 8 | 12 | 16 | 20 | 30 | 40 | 50 | 60 | 80 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mm | 4.75 | 3.35 | 2.36 | 1.70 | 1.18 | 0.85 | 0.60 | 0.425 | 0.30 | 0.25 | 0.18 |
The air resistance in the filter changes depending on the particle size category, provided the carbon volume remains the same. That makes air resistance a key parameter in selecting the right particle size category.
Activated Carbon in Various Types of Filters
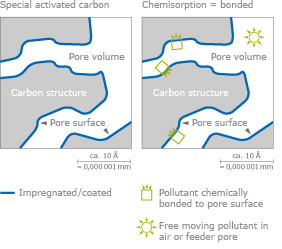
Impregnation
Normal activated carbon removes many odorous substances from the air, including organic solvents or poisons.
However, some common gaseous chemicals such as cyanides or ammonia are adsorbed poorly, or not at all. The activated carbon must be impregnated to eliminate these poisons. Therefore, the large internal surface is coated with reactants for these poisons = chemisorption.
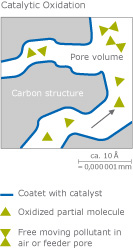
Another group of chemicals can be removed with catalysts (for example phosphines). In this case, the activated carbon is coated with these catalysts. A catalyst promotes a chemical reaction without its own properties changing. Its sole function is to facilitate the reaction.